Allylic And Vinylic Hydrogens
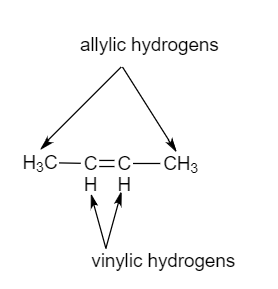
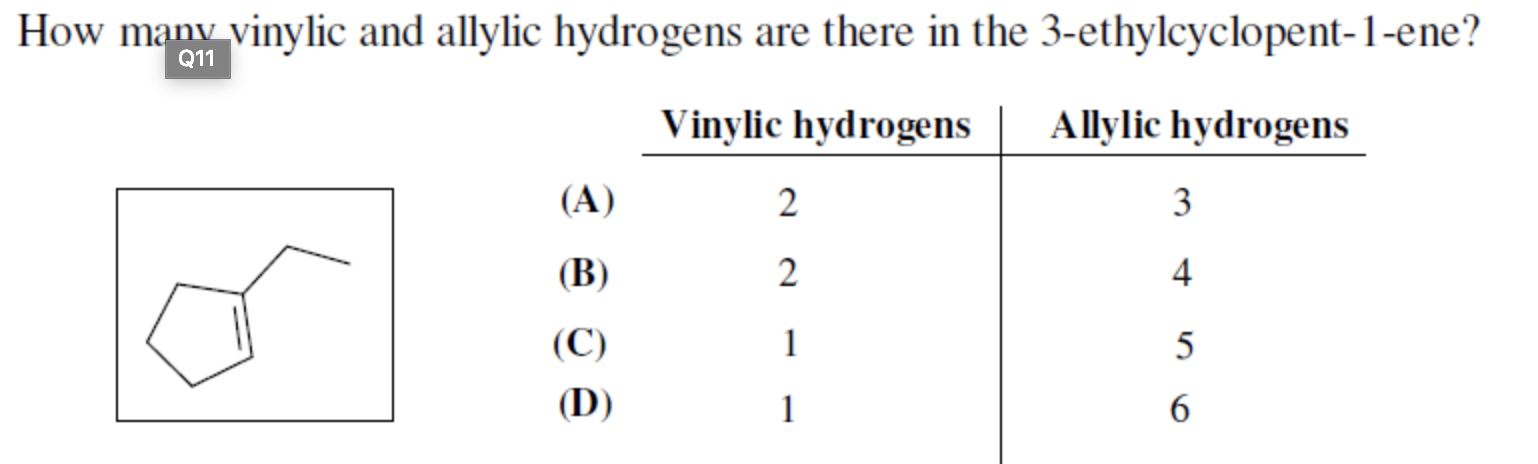
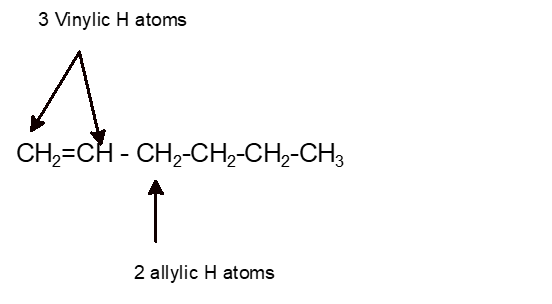
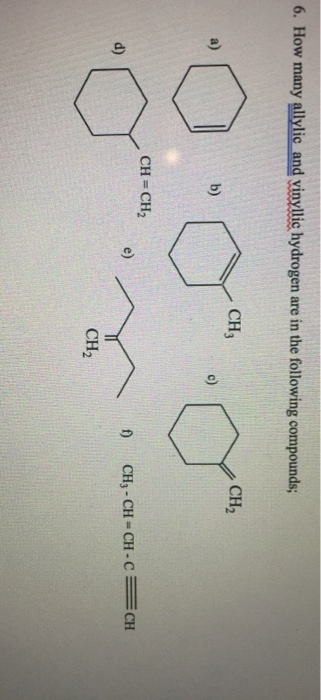
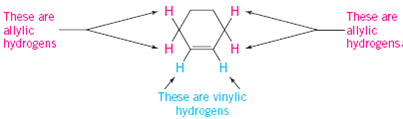
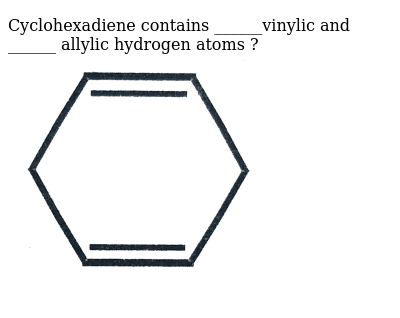
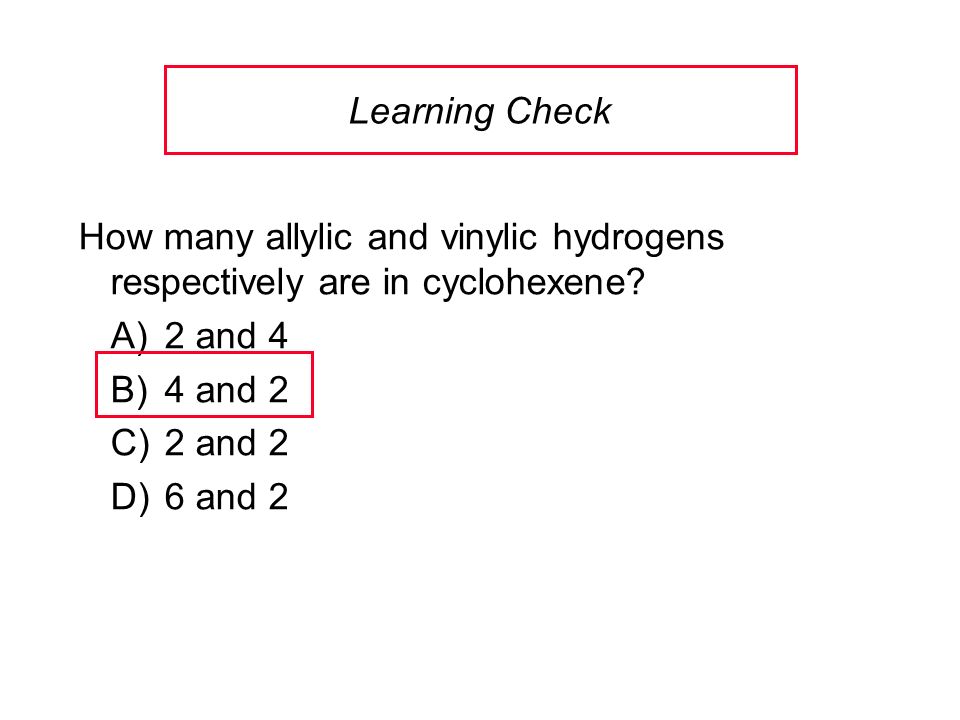
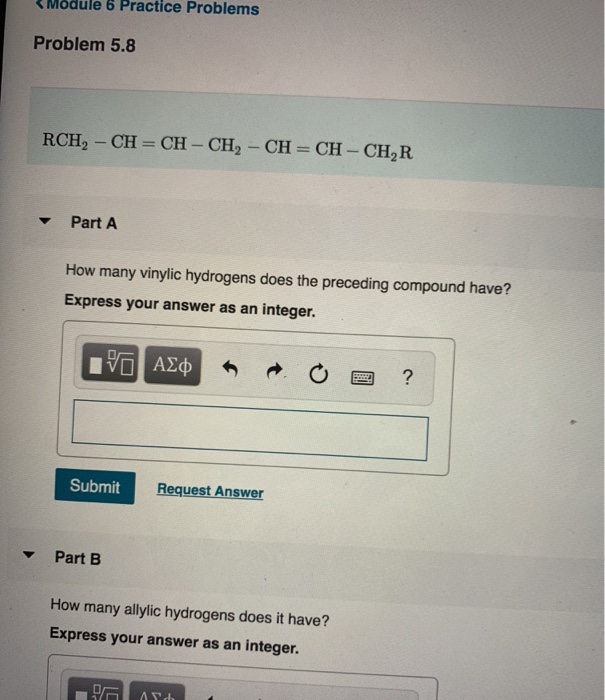
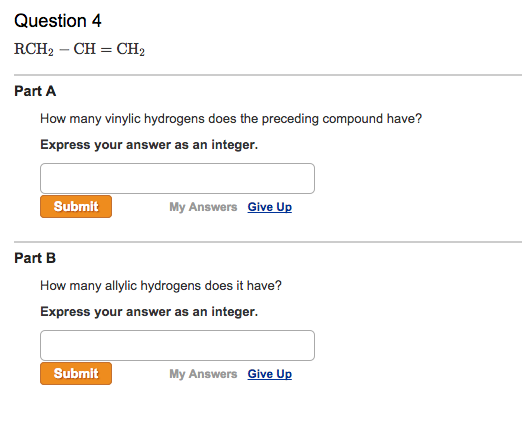
Identify the number of allylic and vinylic hydrogens in the pictured molecules.
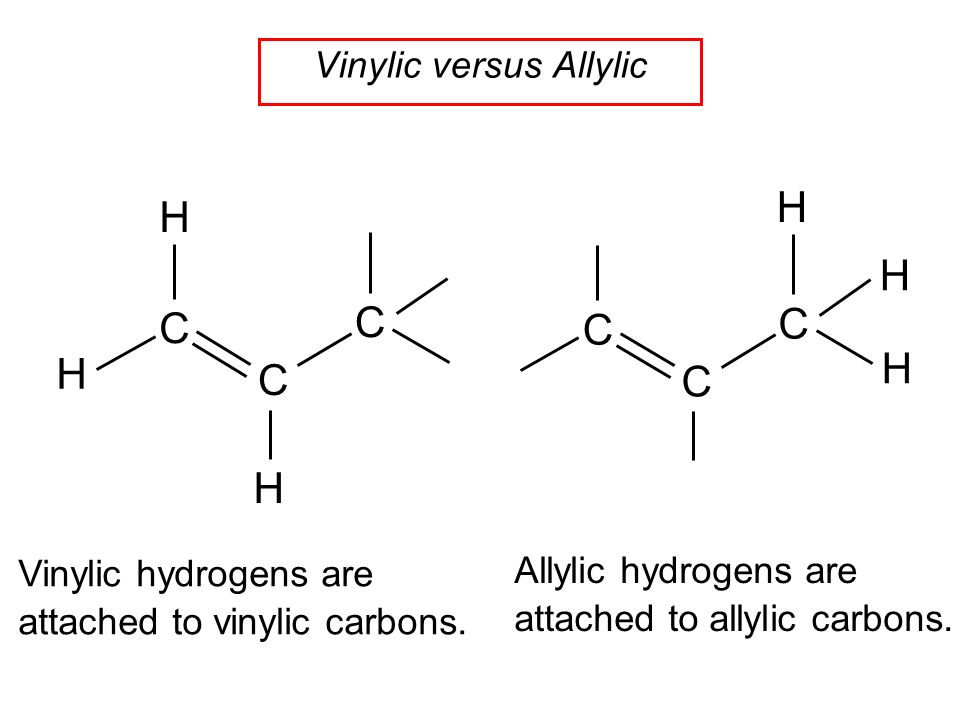
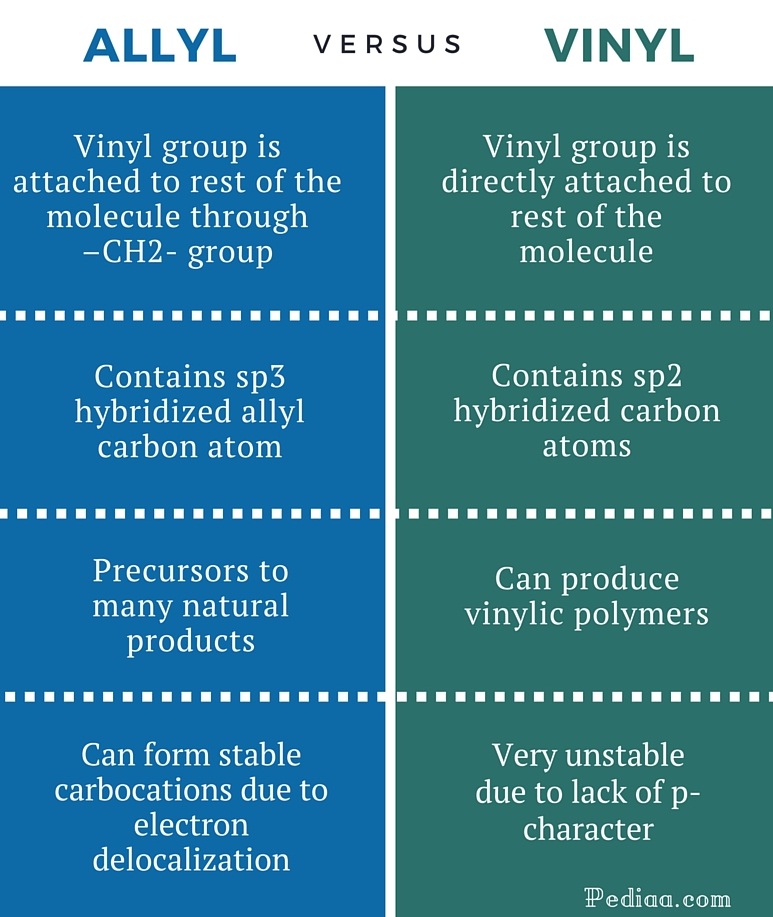
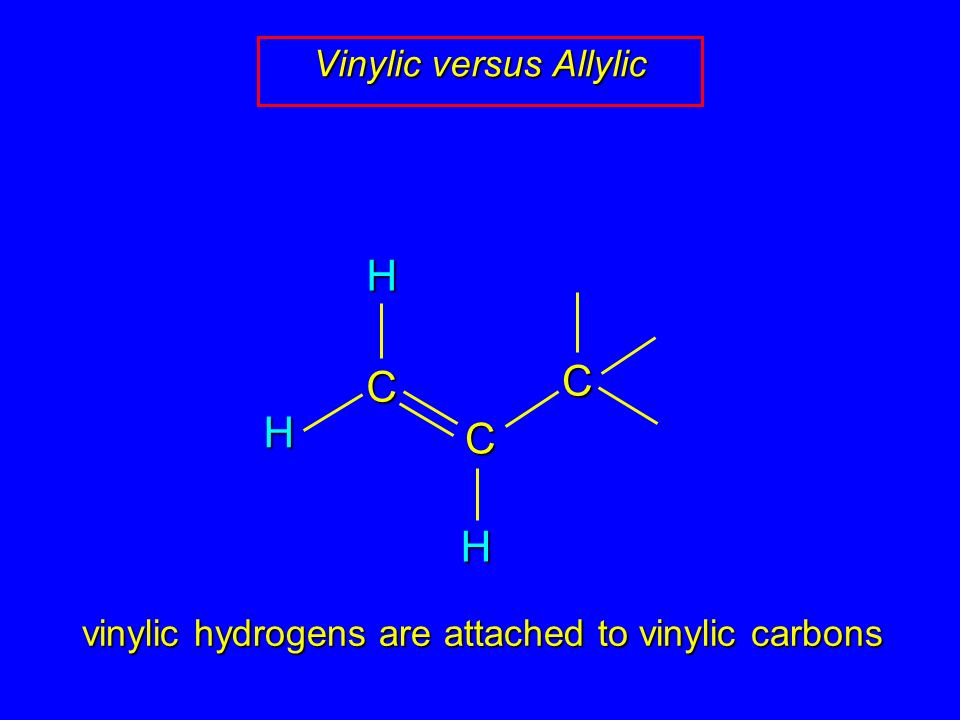
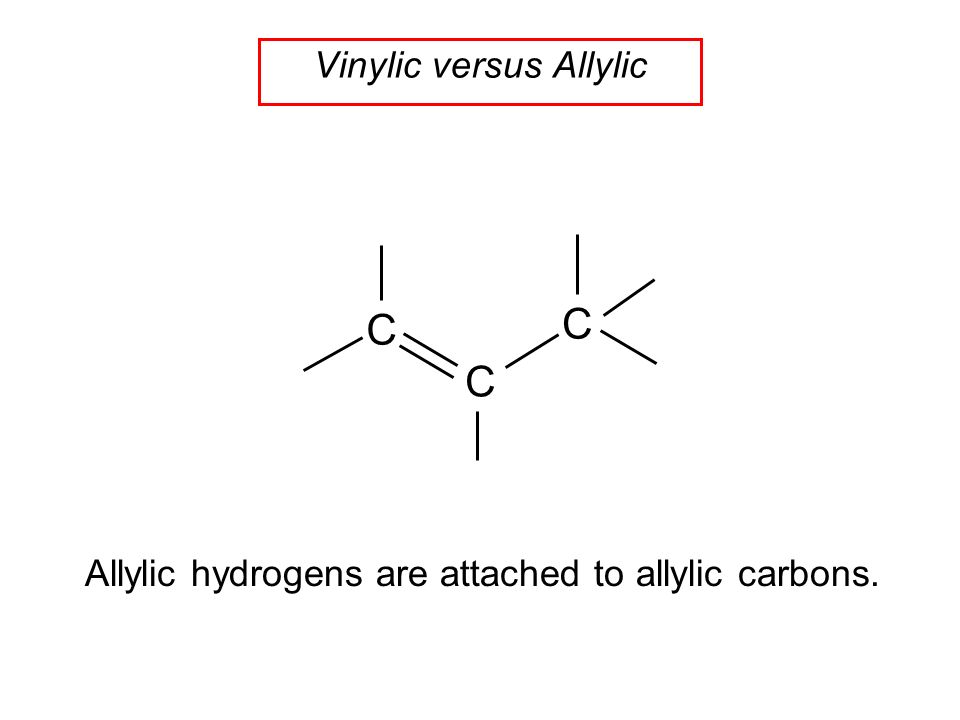
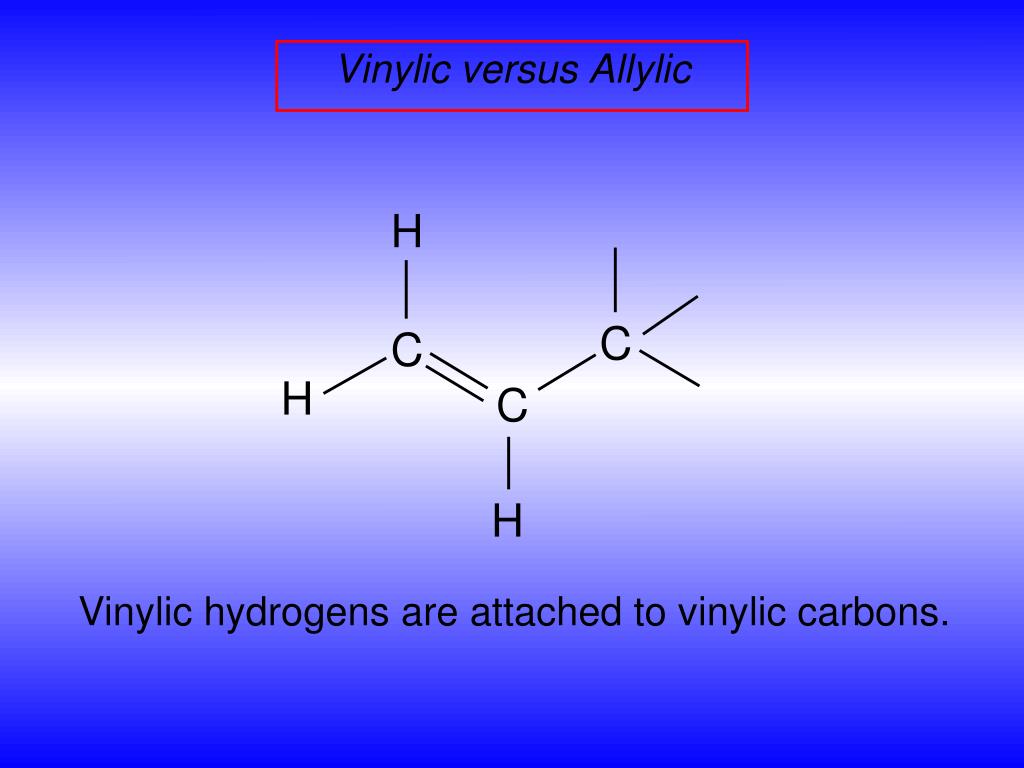
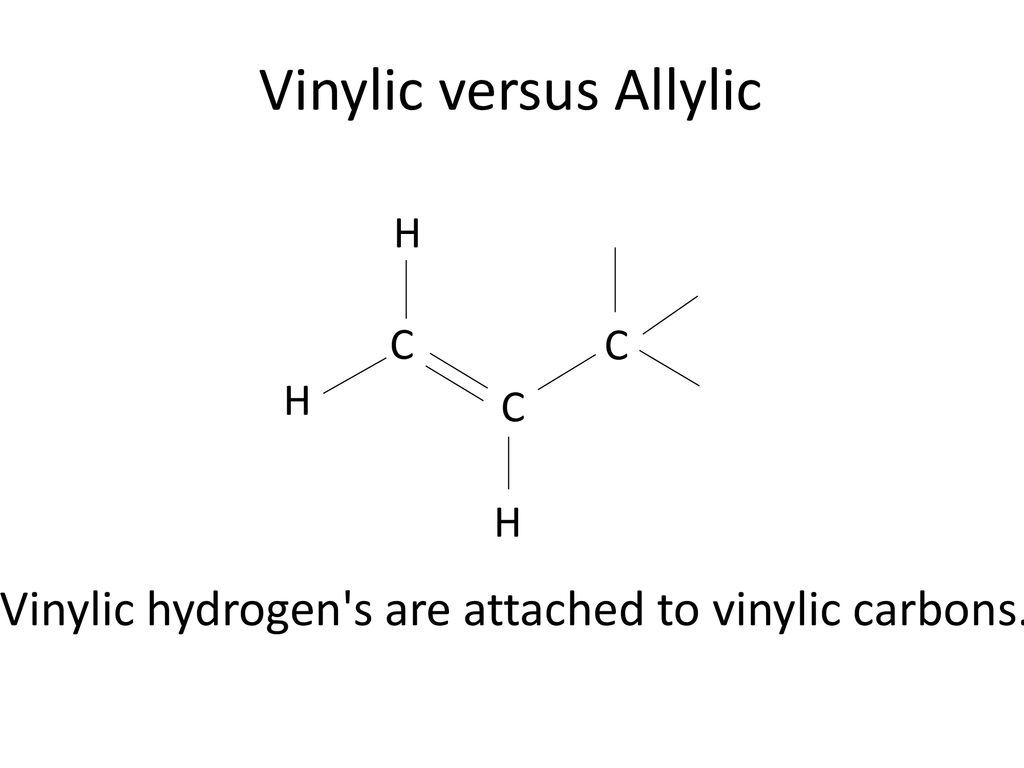
Allylic and vinylic hydrogens. When one hydrogen atom is removed from the third carbon atom of a propane molecule it is equivalent to an allyl group. In other words it is a methylene bridge ch 2 attached to a vinyl group ch ch 2. The key difference between allylic and vinylic carbon is that allylic carbon is the carbon. Allyl group holds three carbon atoms and five hydrogen atoms on the other hand vinyl group has two carbon atoms and three hydrogen atoms.
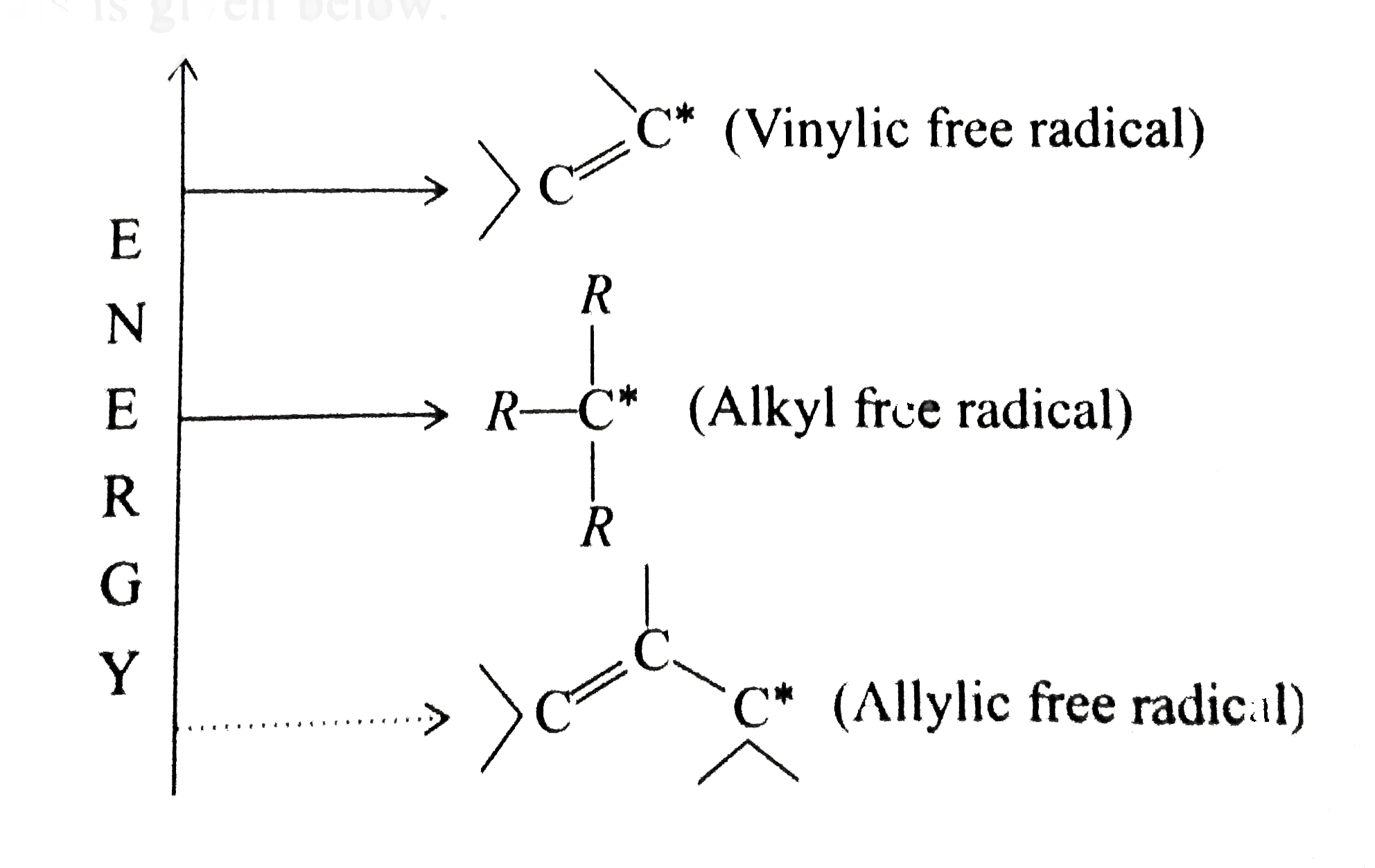
We also acknowledge previous national science foundation support under grant numbers 1246120 1525057 and 1413739. It contains two sp 2 hybridized carbon atoms and one sp 3 hybridized carbon atom. A hydrogen atom bonded to an sp 2 carbon of an alkene. An allylic carbon is an sp3 carbon that is adjacent to a vinylic carbon.
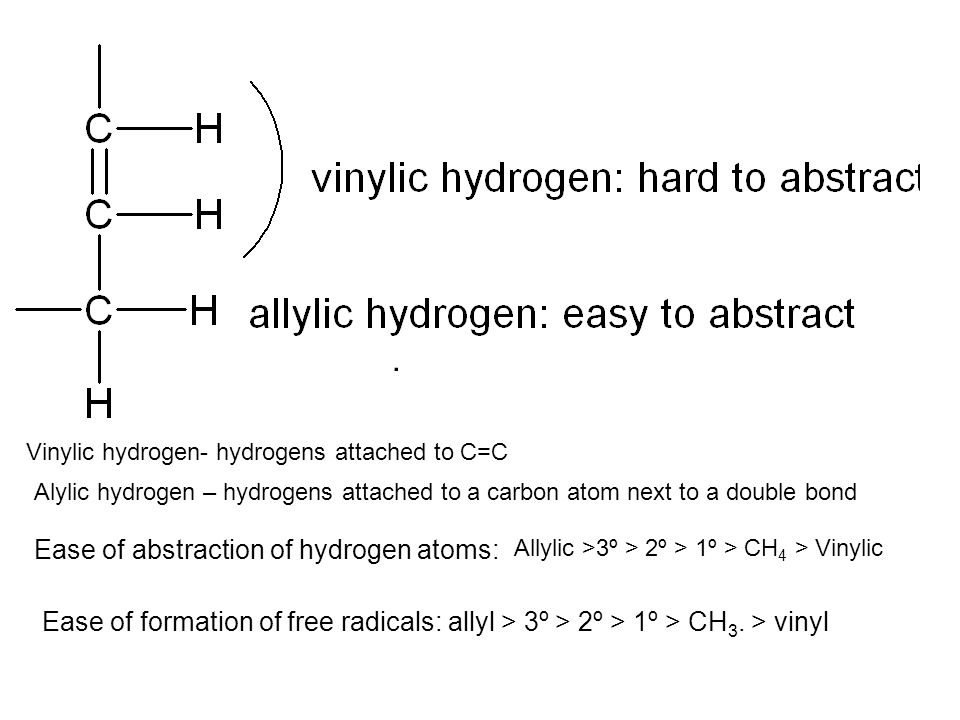
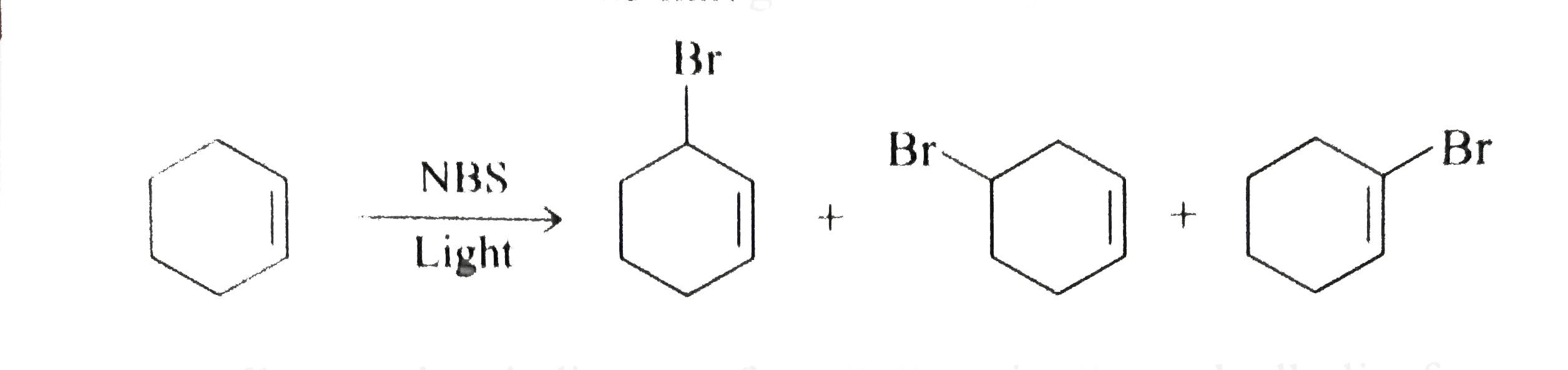
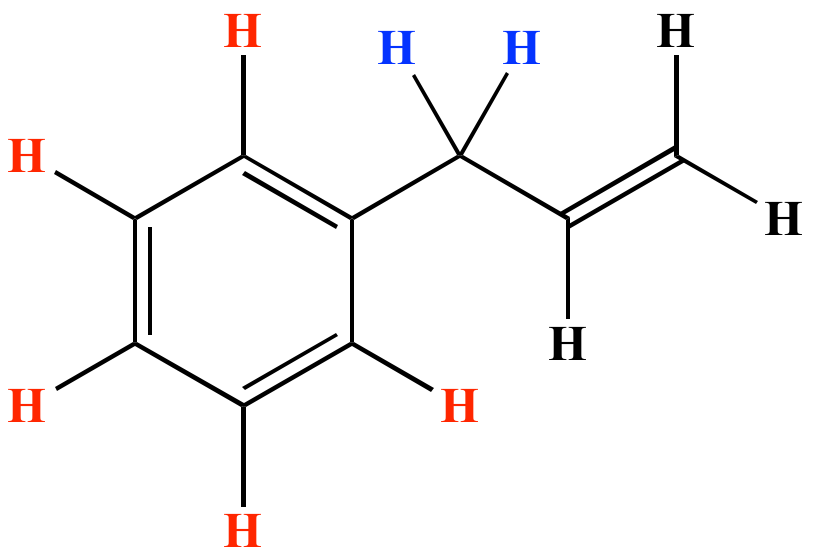
Br allyl radical is stabilished by resonance. Benzylic position allylic position propargylic position aryl aryl hydrogen. The vinylic hydrogens are shown in red. None of the other hydrogens are vinylic.
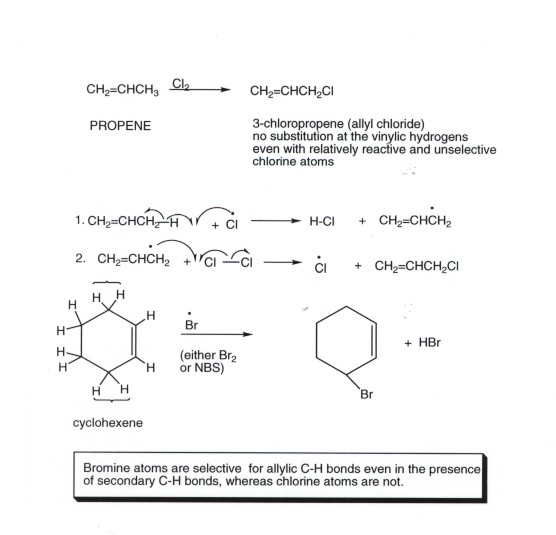
An allylic hydrogen is a hydrogen atom that is bonded to an allylic carbon in an organic molecule. Chlorination of allylic hydrogen is difficult than vinylic hydrogen. Allylic vinylic examples organic chemistry duration. Atoms or groups attached to an allylic carbon are termed allylic substituents.
An allylic carbocation in which an allylic carbon bears the positive charge. Identify the number of allylic and vinylic hydrogens in the pictured molecules. Allyl form a stable carbocation because of the electron delocalization whereas vinylic carbocations are unstable as they lack p character.